Please select your Region.
Please select your Region.
Oct 17, 2016
It is considered that circulating tumor cells (hereafter 'CTCs'), which exist in extremely minute quantities in peripheral blood, enter a blood vessel from a primary tumor and then cause metastasis by circulating around the body.
For cancer diagnosis generally a biopsy is conducted, in which a part of the tissue is collected directly. However, this causes significant strain on the patient. As a result there are high expectations for the establishment of a method that would enable a minimally invasive blood test to detect and analyze CTCs, which in turn would allow for the early detection of cancer as well as the detection of metastasis. Also, the genetic analyses of CTCs for purposes such as checking the drug resistance of tumor cells during anti-cancer therapy are useful as liquid biopsies, which have been gaining attention in recent years as minimally invasive tests.
Despite this, in 10mL of blood there are only around several to several tens of CTCs and as a result there have been significant technical difficulties with the detection of CTCs with regards to accuracy and reproducibility. Moreover, it is considered that the following 3 issues must be addressed in order to detect CTCs with high sensitivity and conduct genetic analysis as a test for clinical application.
1) A large amount of blood cells remain in the sample after processing,
2) Only CTCs with certain markers can be captured,
3) The work is time-consuming and the process itself is considerably laborious.
The research group established a new optimum system for the genetic analysis of CTCs that overcomes these 3 issues.
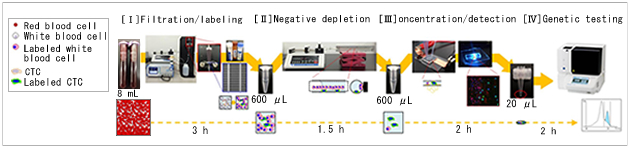
The CTC analysis system that was developed from this research is made up of the following 4 steps.
1) Whole blood is filtered and the cells that are trapped on the filter are immunostained and magnetically labeled.
2) The white blood cells remaining in the cell suspension recovered from the filter are removed and purified.
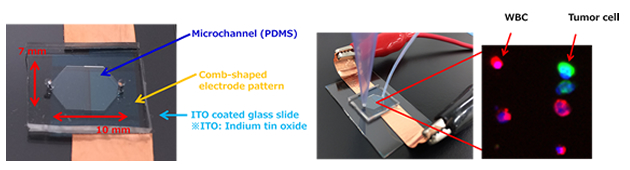
3) The purified cells are enriched at an observation chamber in a microfluidic enrichment device using dielectrophoresis and observed with fluorescence microscopy.
4) All cells enriched in the chamber are recovered and genetic mutations are analyzed with the genetic analysis system i-densy, developed by ARKRAY, without nucleic acid purification.
In order to substantiate the performance of the system, whole blood spiked with cultured cancer cell lines (NCI-H2228, NCI-H1650, NCI-H1975, SW620 or MCF-7) were used and the genetic mutations of the spiked cells (EML4-ALK, EGFR, KRAS or PIK3CA) were detected. As a result, the genetic mutations of each cultured cancer cell line spiked in 8mL of whole blood were successfully detected. The detection sensitivity of this method is 1 cell/mL and at this concentration the success rates for detection of cancer cells and the genetic mutations (of those cells) were 100% (10/10 tests). Furthermore, the average number of residual white blood cells was 87 and the system could report the results of genetic mutation and each enumeration of cancer cells and residual white blood cells within 9 hours of starting the processing of whole blood.
Additionally, this system does not sort CTCs with specific markers and incorporates a method of removing only unnecessary blood cells. Therefore, the genetic mutations of various types of CTCs are detectable and as a result the system may also be effective for CTCs that have changed their phenotype.
From the results above, it can be concluded that use of the system enables simple and fast analysis of CTC genetic mutations.
In cancer treatment early detection and the selection of an effective treatment method is crucial, as is predicting the chances of metastasis or recurrence. In this respect, CTC testing is expected to serve as an effective measure for cancer treatment and ongoing progress has been made in clinical research. ARKRAY shall further accelerate initiatives aimed at the creation and practical application of new testing technologies in order to increase access to minimally invasive and more effective cancer treatments at the frontlines of clinical treatment and provide such treatments to even more patients.
Complete process diagram
Concentration device
The i-densy IS-5320 is an instrument for the fully-automatic analysis of base pair sequences associated with drug metabolism and cancer related genetic mutations. It does not require a high degree of technical skill to operate and all steps from sample pre-treatment to gene amplification and genotyping are simple. Where previously it took many days from sample collection to output of results, genotyping now takes 80 minutes. In addition to the IS-5320, ARKRAY also developed and sells the in vitro genetic reagents i-densy Pack UGT1A1 (*28/*6) and i-densy Pack CYP2C19 (*2/*3), and we continue to actively undertake the development of products that support personalized medicine.

![]()